Following on the heels of the successful statewide Good Catch Campaign in 2017, American Data Network Patient Safety Organization (ADNPSO) is launching a year-long specimen event study across 15 hospitals in November 2018. The intent of the study, which is free to members of ADNPSO, is to help healthcare professionals, both inside and outside the laboratory, better understand why specimen events happen and how they can collaborate to decrease errors across the testing process.
Laboratory testing provides essential information used by providers in medical decision making with an estimated 60–70% of these decisions based on laboratory test results (Green 2013). Patient safety events involving specimens can be precursors to serious mistakes, including diagnostic errors and inappropriate treatments. Needless to say, Specimen events can have a dramatic impact on patient care and satisfaction, as well as a hospital’s quality, safety and financial outlook.
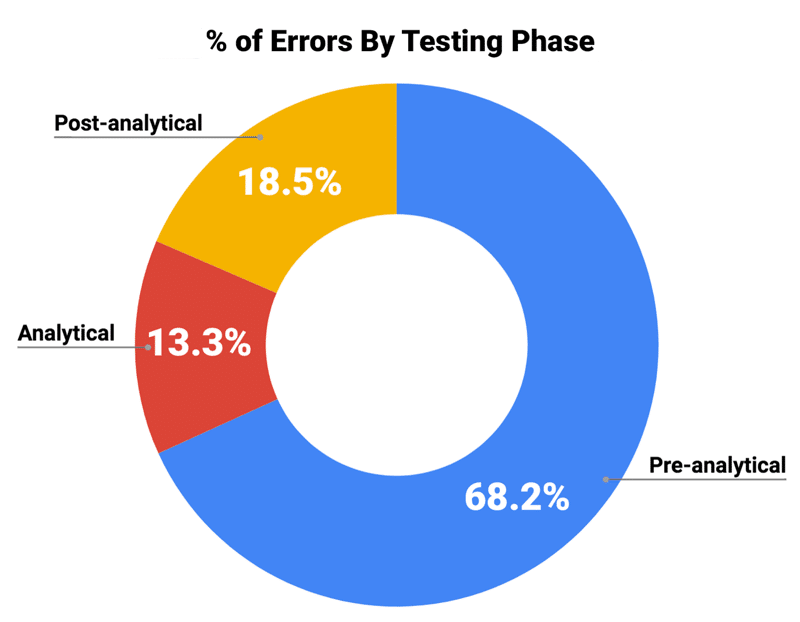
While mix-ups and mishaps do happen in laboratories, literature suggests that the majority of specimen events actually occur outside the lab. In fact, errors occurring in the pre-analytical phase of testing, like mislabeling and inaccurate patient identification, account for 68.2% of all specimen events. Studies also find that events occurring in the post-analytical phase are more likely to result in patient harm, like when critical results are not reported in a timely manner.

Specimen errors can be expensive, too. Researchers at Loyola University Health System calculated the average cost of a labeling error to be $712 per redraw, not including inestimable damages of patient anxiety, discomfort and delays in diagnosis or treatments. Consider a hospital with an annual specimen labeling defect rate of 0.02% per million tests. This would result in 2,000 patients requiring redraws and cost the hospital over $1.4 million.
The good news is that specimen errors are believed to be highly preventable, and effective interventions based on strong policies, clear feedback, and relevant education do not need to be resource intensive. After analyzing nearly 3,000 specimen events collected over four years, ADNPSO noted that 77% of specimen incidents either almost certainly or likely could have been prevented.
Standardizing the Specimen Data Collection
Many event reporting tools and processes categorize specimen events as “Other,” making data analysis difficult. ADNPSO collaborated with other Patient Safety and Laboratory experts to design a proprietary Specimen Reporting Form. Standardizing the specimen form allows for collection of the same discrete data elements across all participating facilities, maximizing the analytic potential and providing clearer insight into root causes. ADN’s specimen form also addresses Near Misses and Unsafe Conditions in addition to actual Incidents. Data elements to be gathered include: Specimen Source, Collection Technique, Collector Details, Error Categorizations, Patient Outcomes and more.
Participants in the Specimen Focused Study will all utilize American Data Network’s electronic event reporting application, the Quality Assurance Communication system, to ensure secure data entry using the standardized Specimen Reporting Form and seamless data transmission to ADNPSO for use in analytics.
Timeline of Study
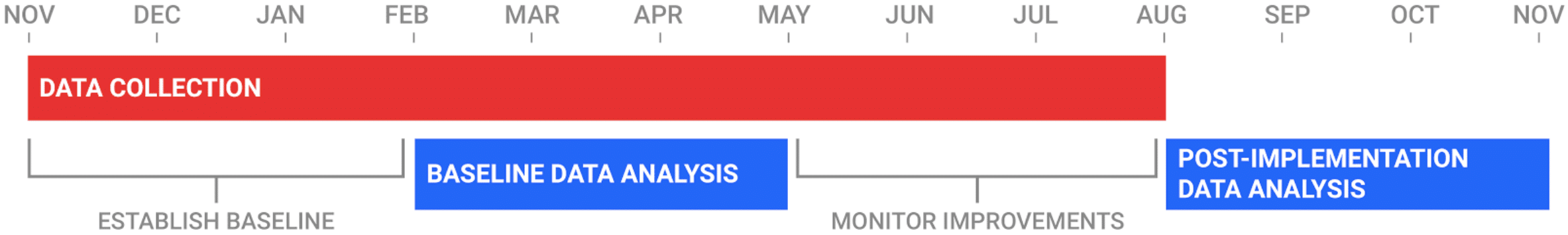
During the first quarter of the study, participating hospitals will submit specimen data for Incidents, Near Misses and Unsafe Conditions to establish a baseline. In the following quarter, ADNPSO will conduct an analysis of the data and provide the hospitals quality/safety improvement strategies. The hospitals will then use the third quarter to implement the improvement recommendations. In the final 3 months of the study, ADNPSO will perform a second analysis to evaluate the effectiveness of the corrective actions, as well as examine overall trends in reporting.
For more information about the Specimen Study or American Data Network Patient Safety Organization membership, contact Susan Allen at sallen@americandatanetwork.com.
Sources:
Goldschmidt HMJ, Lent RW. Gross errors and work flow analysis in the clinical laboratory. Klin Biochem Metab 1995;3:131–40.
Green, S. F. (2013). The cost of poor blood specimen quality and errors in preanalytical processes. Chemical Biochemistry, 46, 1175-1179.
Kahn S, Jarosz C, Webster K, et al. Improving process quality and reducing total expense associated with specimen mislabeling in an academic medical center. Poster. 2005 Institute for Quality in Laboratory Medicine Conference: Recognizing Excellence in Practice. April 28, 2005. Accessed September 21, 2009.
Plebani M, Carraro P: Mistakes in a stat laboratory: Types and frequency. Clin Chem 1997;43:1348-1351.




